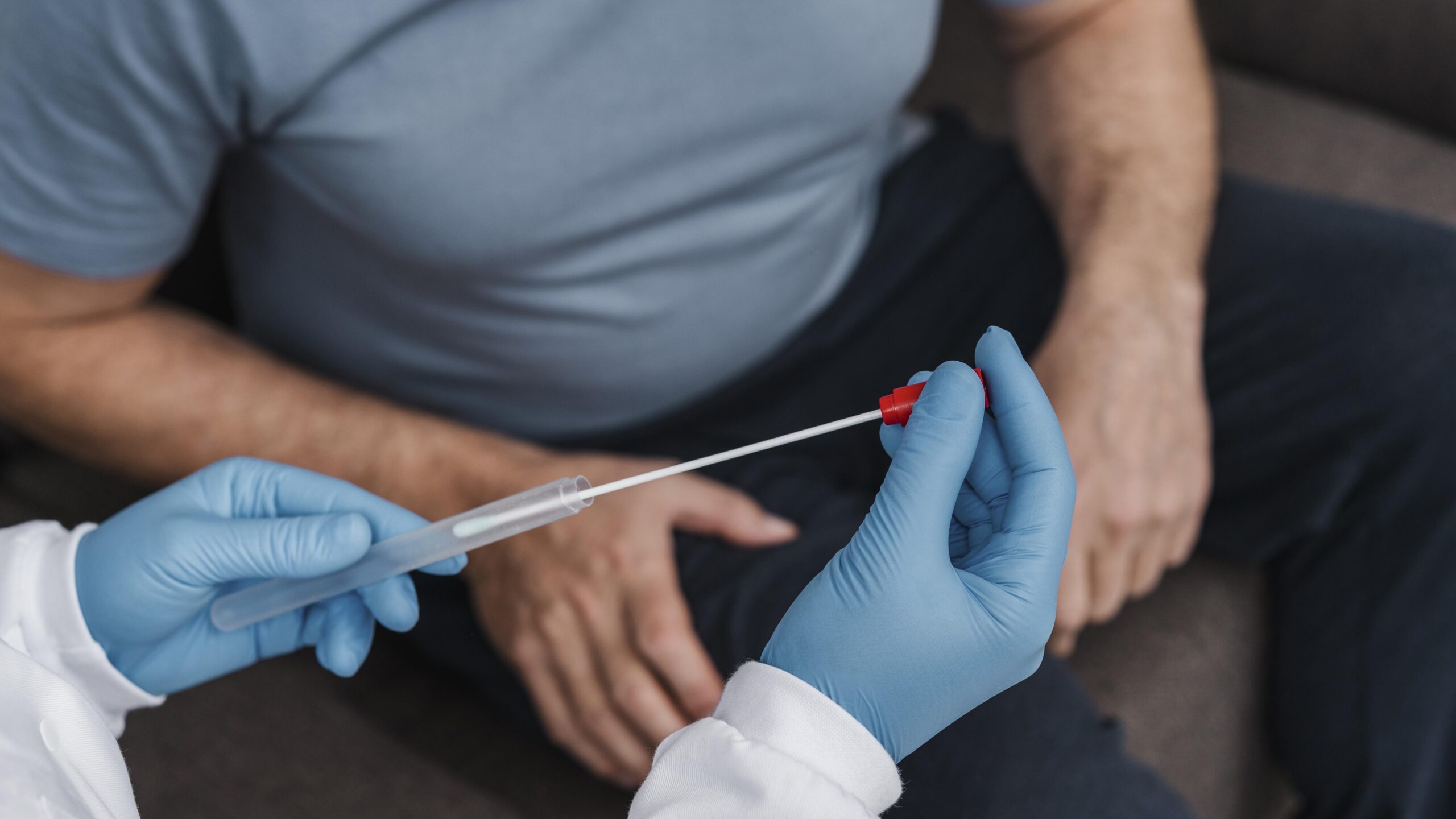
A man considered healthy donated sperm through a European sperm bank, unknowingly carrying a rare genetic mutation linked to a high risk of cancer. His donations resulted in the births of at least 67 children across multiple countries. Years later, 10 of those children have been diagnosed with cancer, and at least 20 more carry the same inherited gene mutation. This case has raised serious questions about how sperm donors are screened, how many families a single donor should be allowed to assist, and how well fertility clinics and banks are equipped to protect future children from inherited medical risks.
The genetic variant involved—TP53—is tied to Li-Fraumeni syndrome, a condition that makes individuals more susceptible to aggressive, early-onset cancers. It’s a well-documented risk, but it wasn’t part of the standard screening when the donor gave sperm between 2008 and 2015. Now, families are left dealing with diagnoses that could have potentially been avoided if more comprehensive safeguards were in place. Experts are calling for changes—not just to screening practices, but to the way donor sperm is distributed, tracked, and regulated internationally.
A Tragic Oversight – How a Rare Mutation Went Undetected

A man who appeared to be in good health donated sperm to a European sperm bank, unaware that he carried a rare genetic mutation in the TP53 gene. This mutation is associated with Li-Fraumeni syndrome (LFS), a condition that drastically increases the risk of developing several aggressive cancers, including leukemia, brain tumors, and sarcomas. Over the span of several years, his sperm was used to conceive at least 67 children. So far, 10 of those children have developed cancer, while another 20 have tested positive for the same genetic variant. The situation first came to light when two separate families contacted fertility clinics after their children were diagnosed with cancer. Genetic testing traced the mutation back to the same donor.
At the time of the donations, most of which occurred between 2008 and 2015, TP53 was not part of routine donor screening. Even though the donor underwent standard health checks and was considered healthy, the mutation remained undetected. Dr. Edwige Kasper, a biologist at Rouen University Hospital, analyzed the TP53 variant using a range of scientific databases and concluded it was very likely cancer-causing.
She urged that children conceived from this donor receive genetic counseling. While full-genome sequencing for all donors isn’t currently feasible or standard practice, experts like Kasper argue that this case underscores how serious health risks can go unnoticed in current fertility screening protocols—especially when a donor’s sperm is used widely.
What makes this case even more troubling is the scale and lack of coordinated oversight. The European Sperm Bank has a limit of 75 families per donor, which is higher than many other regions, and in this case, children were conceived across multiple countries. This cross-border use complicates efforts to trace and inform affected families, many of whom may still be unaware of the inherited risk. Experts like Professor Nicky Hudson of De Montfort University point out that without international tracking systems and stricter usage limits, similar cases could happen again. The European Sperm Bank has acknowledged the issue and stated that it’s not currently possible to detect every potentially harmful mutation unless you know exactly what to look for. However, the damage is already done for the families affected—and the incident has sparked urgent calls for reform.
Gaps in Genetic Screening – What Current Protocols Miss

Sperm banks routinely screen donors for a variety of infectious diseases, inherited conditions, and certain genetic markers. But these screenings are far from exhaustive. Most banks use targeted panels that focus on common, well-known conditions—such as cystic fibrosis, Tay-Sachs, or spinal muscular atrophy—rather than conducting full-genome sequencing. In the case of the TP53 mutation, it wasn’t included in standard panels at the time of the donations. This isn’t unusual. Many rare but high-risk mutations don’t make it into routine screenings because they’re either considered too uncommon, too expensive to test for broadly, or not well understood at the time.
The limitations of current testing protocols raise a critical question: how many other rare mutations are going undetected in otherwise “healthy” donors? While genome sequencing is becoming more accessible and cost-effective, it’s not yet standard in reproductive medicine. And even when genetic data is available, interpreting its clinical significance can be challenging.

Not all mutations have clear outcomes or risk profiles, and identifying a variant doesn’t always mean it will lead to disease. That said, the TP53 mutation linked to Li-Fraumeni syndrome is one of the more well-documented, with strong evidence tying it to early-onset cancers. Experts like Dr. Kasper and others argue that including high-penetrance genes like TP53 in donor screening could help prevent severe outcomes like the ones seen in this case.
Another major challenge is timing. Some conditions tied to genetic mutations don’t appear until later in life, meaning a donor might seem healthy and symptom-free for years while unknowingly passing on serious risks to their biological children. This underscores a flaw in the assumption that a healthy donor today is a safe donor for the future. As reproductive technologies continue to advance, the screening protocols must keep up. Otherwise, families may unknowingly inherit genetic risks that could have been flagged—and potentially prevented—if more comprehensive testing had been in place.
The Problem with Donor Limits and Cross-Border Fertility

One of the most alarming aspects of this case is the sheer number of children conceived from a single donor—at least 67, and possibly more. The European Sperm Bank, where the donations took place, currently allows up to 75 families per donor. This is significantly higher than the limits set in many other countries. For example, the UK restricts donor sperm to 10 families, and the Netherlands limits it to 12. The higher the number of families, the greater the risk of widespread transmission if a donor carries a serious genetic condition. In this case, the consequences of that policy became painfully clear.
Beyond the numbers, international use of donor sperm adds another layer of complexity. Sperm from this donor was used across multiple countries, making it extremely difficult to trace and notify all recipient families once the TP53 mutation was discovered.

Professor Nicky Hudson has highlighted this issue, noting that while such cases are rare, the fragmented and uncoordinated nature of cross-border fertility treatment makes it hard to implement consistent safeguards. Different countries have different regulations, databases, and follow-up protocols, which means that when something goes wrong, there’s no unified system to respond. Families may remain unaware of inherited risks simply because there’s no efficient way to reach them.
This case has prompted renewed calls for an international limit on the number of births per donor, not just within a single sperm bank or country. Several experts, including those affiliated with the European Sperm Bank itself, have supported this idea. They’ve also emphasized the need for better tracking systems that allow clinics to monitor donor usage across borders. Without these changes, the current system remains vulnerable—not just to medical oversights, but also to a lack of accountability when things go wrong. Reforming these policies isn’t just a logistical matter; it’s a public health necessity.
What Prospective Parents Can Do – Practical Tips for Safer Donor Selection

For those considering donor sperm, the recent case involving the TP53 mutation is a stark reminder that not all health risks can be screened out under current protocols. While most sperm donors are tested for a core set of genetic and infectious diseases, these panels vary widely between clinics and often exclude less common but high-impact mutations. That means prospective parents need to go beyond surface-level reassurances and ask detailed, specific questions. Start by requesting a complete list of the conditions each donor has been screened for, including whether any cancer-related genes—like BRCA1/2 or TP53—are part of the panel. Transparency is key, and a reputable clinic or bank should be open about what they do and don’t test for.
In some cases, it may be worth choosing a clinic that offers expanded carrier screening or even full-exome or genome sequencing of donors. These broader tests can identify a wider range of genetic risks, including those not covered by traditional panels. While this type of screening might not be standard or inexpensive, it could provide important peace of mind—especially for individuals with a family history of cancer or other inherited diseases.
Even if a donor appears to be in excellent health, that alone isn’t enough to rule out the presence of silent genetic mutations. Asking about long-term donor tracking systems and whether the clinic notifies recipients of newly discovered donor-related health issues can also be an important safeguard.
Equally important is involving a genetic counselor in the decision-making process. Genetic counselors are trained to interpret complex data, help you understand inherited risks, and recommend additional testing where appropriate. They can also assess your own genetic background, which can be just as important when selecting a compatible donor. A one-time consultation could help you make more confident and informed choices. Ultimately, while fertility clinics and sperm banks bear the primary responsibility for screening donors, prospective parents have a critical role to play in asking informed questions, demanding transparency, and seeking professional guidance to reduce avoidable risk.
Why This Case Should Change the Industry – A Call for Reform

The TP53 case is not just an isolated tragedy—it’s a warning signal that current sperm donation practices, particularly at the international level, are falling short of basic safeguards. When a single donor’s sperm leads to the conception of dozens of children across borders, without a system in place to track or recall affected families, it creates a public health gap that’s too dangerous to ignore. The existing patchwork of regulations—where each country and sperm bank sets its own limits and policies—has created a loophole where serious genetic risks can go undetected and unaddressed. The fact that this donor passed on a known cancer-related mutation to at least 30 children, 10 of whom already have cancer, should be enough to prompt structural change.
Industry experts have already called for action. Biologists like Dr. Edwige Kasper and public health researchers have stressed the need for internationally coordinated limits on donor usage. A cap on the number of families per donor—enforced across all countries, not just within individual banks—could significantly reduce the impact of future cases like this. In parallel, better systems for monitoring, communication, and follow-up must be developed. That includes maintaining up-to-date contact records for donor-conceived families and creating rapid response systems when a health issue arises. Clinics should also be required to report and share medically relevant outcomes that emerge post-donation, rather than leaving families to discover them independently.
For now, families impacted by this case are left grappling with the consequences—some facing childhood cancer, others living with the knowledge that their children carry a high-risk mutation. But the broader lesson is clear: donor conception is not just a medical transaction; it’s a long-term responsibility. It’s time for fertility clinics, regulators, and international bodies to stop treating genetic risk as an exception and start building systems designed to catch what current protocols miss. The technology exists. The evidence is here. What’s needed now is the will to change how donor screening, tracking, and family limits are handled—before another preventable tragedy unfolds.






Leave a Reply